Onvansertib
Onvansertib effectively targets PLK1
PLK1 is an enzyme over-expressed in many cancer types. It’s been known for decades to to be hijacked by tumor cells allowing uncontrolled growth in the M phase of the cell cycle. We now know PLK1 is involved in the repair of damaged DNA in the S phase of the cell cycle.
As chemotherapies and targeted cancer drugs damage DNA, onvansertib inhibits PLK1’s ability to repair DNA damage and increases the efficacy of the standard of care therapy in a variety of indications.
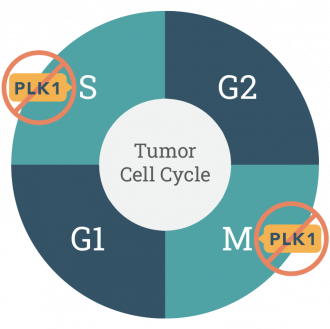
Onvansertib's characteristics support its safety and efficacy profile
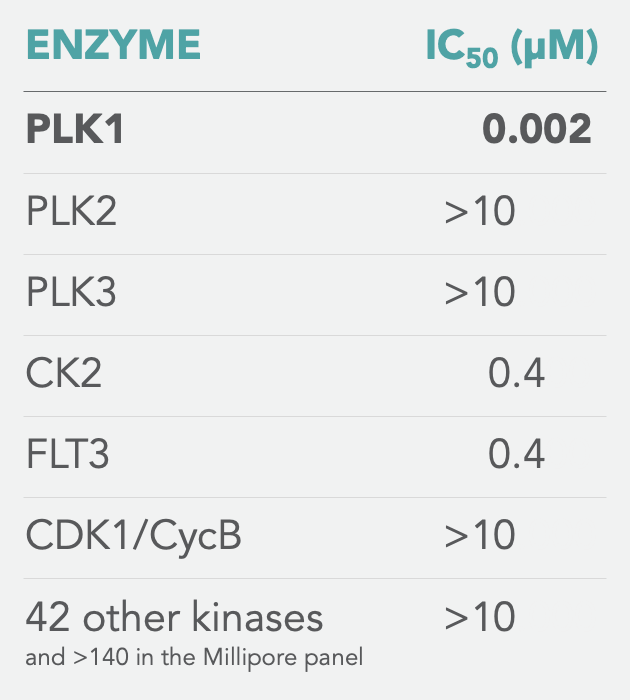
SPECIFICITY
Onvansertib is exquisitely specific for PLK1
PROPERTIES
Onvansertib is an orally dosed small molecule with a 24-hour half-life
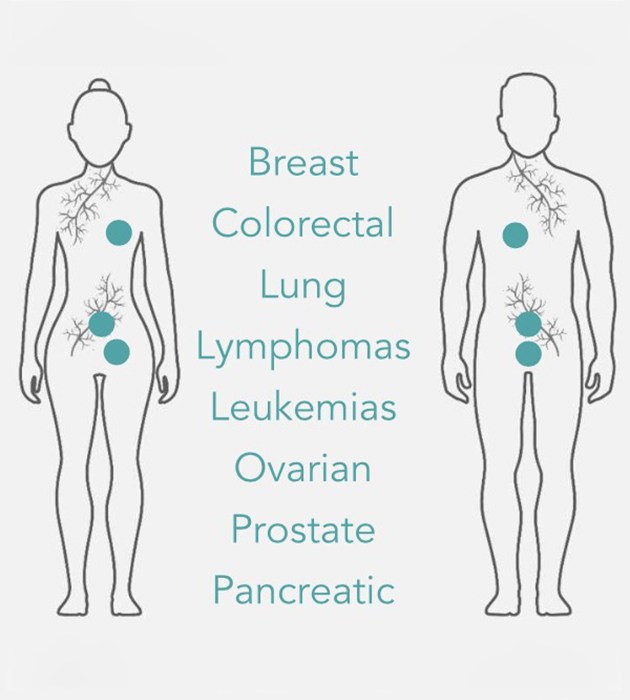
OPPORTUNITY
PLK1 is over-expressed in many cancer types
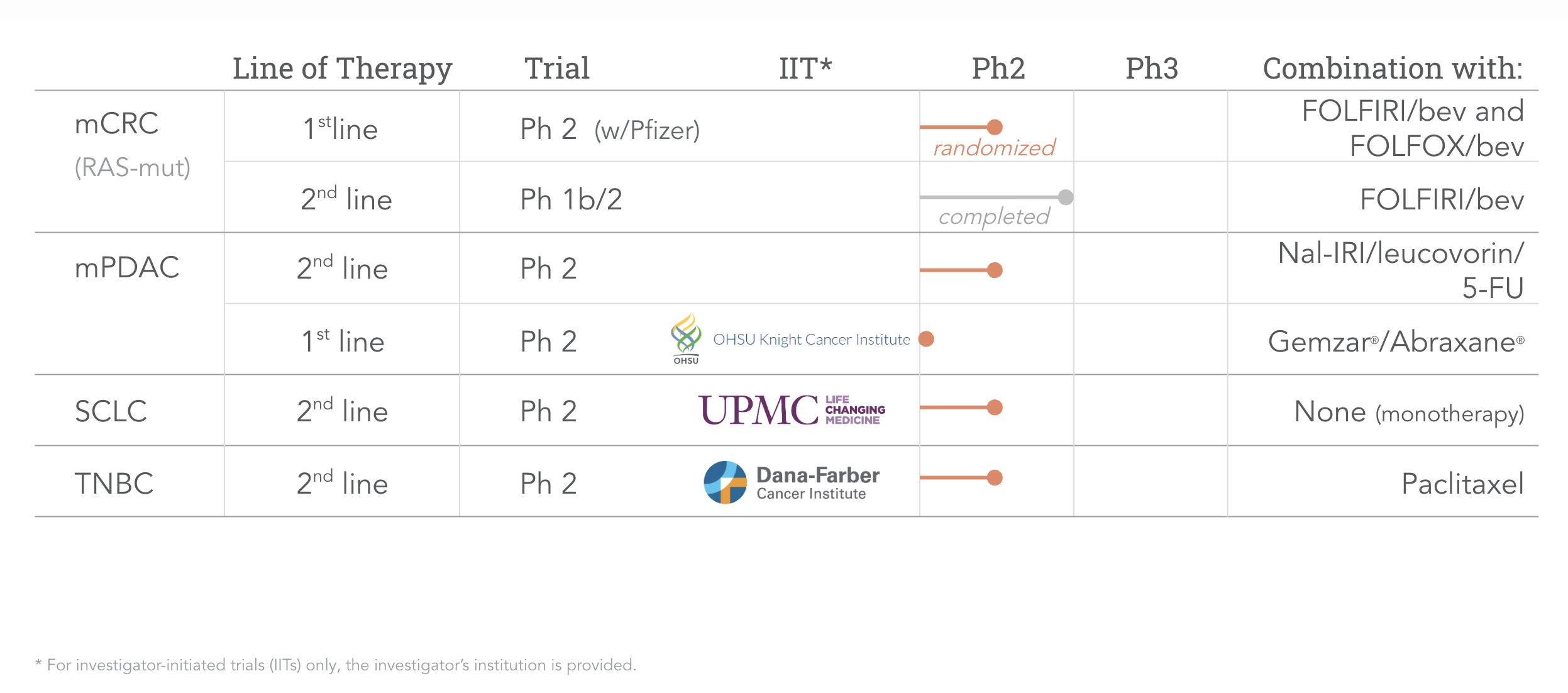
Our pipeline opens many attractive opportunities for onvansertib
Looking to partner with us?
Please send inquiries to [email protected]
